The time required for 10% completion of a first order reaction at 298 K is equal to that required for its 25% completion at 308 K. If the value of A is 4 x 1010 s-1. Calculate k at 318 K and Ea.
For a first order reaction,
t = 2.303 / k log a / a - x
At 298 K,
t = 2.303 / k log 100 / 90
= 0.1054 / k
At 308 K,
t' = 2.303 / k' log 100 / 75
= 2.2877 / k'
According to the question,
t = t'
⇒ 0.1054 / k = 2.2877 / k'
⇒ k' / k = 2.7296
From Arrhenius equation,we obtain
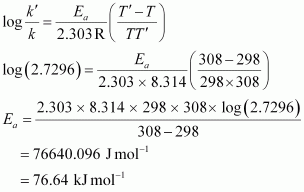
To calculate k at 318 K,
It is given that, A = 4 x 1010 s-1, T = 318K
Again, from Arrhenius equation, we obtain

Therefore, k = Antilog (-1.9855)
= 1.034 x 10-2 s -1
Popular Questions of Class 12 Chemistry
- Q:-
Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone
(vii) Ethanal and Propanal
- Q:-
A 5% solution (by mass) of cane sugar in water has freezing point of 271 K. Calculate the freezing point of 5% glucose in water if freezing point of pure water is 273.15 K.
- Q:-
How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
- Q:-
A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of solution is 1.2 g mL-1, then what shall be the molarity of the solution?
- Q:-
Henry's law constant for CO2 in water is 1.67 x 108Pa at 298 K. Calculate the quantity of CO2in 500 mL of soda water when packed under 2.5 atm CO2 pressure at 298 K.
- Q:-
Calculate the mass of a non-volatile solute (molar mass 40 g mol-1) which should be dissolved in 114 g octane to reduce its vapour pressure to 80%.
- Q:-
The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively, at 350 K. Find out the composition of the liquid mixture if total vapour pressure is 600 mm Hg. Also find the composition of the vapour phase.
- Q:-
Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
- Q:-
How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of both?
- Q:-
If NaCl is doped with 10-3mol % of SrCl2, what is the concentration of cation vacancies?
Recently Viewed Questions of Class 12 Chemistry
- Q:-
Give the IUPAC names of the following compounds:
(i) PhCH2CH2COOH (ii) (CH3)2C=CHCOOH
(iii)
 (iv)
(iv) 
- Q:-
Explain briefly how +2 state becomes more and more stable in the first half of the first row transition elements with increasing atomic number?
- Q:-
Why do soaps not work in hard water?
- Q:-
How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
- Q:-
Write the mechanism of the reaction of HI with methoxymethane.
- Q:-
The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 Scm-1. Calculate its molar conductivity.
- Q:-
Depict the galvanic cell in which the reaction Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s) takes place.
Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
- Q:-
If NaCl is doped with 10-3mol % of SrCl2, what is the concentration of cation vacancies?
- Q:-
Why are halogens coloured?
- Q:-
List the important sources of sulphur.
4 Comment(s) on this Question
Wonderful. I appreciate your efforts. But you can make this visually appealing too.
Helps me a lot
Thanks for clearing my doubts
I thought it's very complicated but it is much easier....thanks for clearing my doubts...
- NCERT Chapter
