Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6]4-
(ii) [FeF6]3-
(iii) [Co(C2O4)3]3-
(iv) [CoF6]3-
(i) [Fe(CN)6]4-
In the above coordination complex, iron exists in the +II oxidation state.
Fe2+ : Electronic configuration is 3d6
Orbitals of Fe2+ ion:

Hence, the geometry of the complex is octahedral and the complex is diamagnetic (as there are no unpaired electrons).
(ii) [FeF6]3-
In this complex, the oxidation state of Fe is +3.
Orbitals of Fe+3 ion:

Hence, the geometry of the complex is found to be octahedral.
(iii) [Co(C2O4)3]3-
Cobalt exists in the +3 oxidation state in the given complex.
Orbitals of Co3+ ion:

Hence, the geometry of the complex is found to be octahedral.
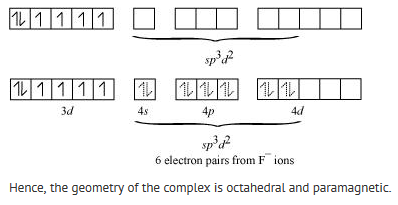
(iv) [CoF6]3- Cobalt exists in the +3 oxidation state.
Orbitals of Co3+ ion:

Again, fluoride ion is a weak field ligand. It cannot cause the pairing of the 3d electrons. As a result, the Co3+ ion will undergo sp3d2 hybridization. sp3d2 hybridized orbitals of Co3+ ion are:
Popular Questions of Class 12 Chemistry
- Q:-
Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone
(vii) Ethanal and Propanal
- Q:-
A 5% solution (by mass) of cane sugar in water has freezing point of 271 K. Calculate the freezing point of 5% glucose in water if freezing point of pure water is 273.15 K.
- Q:-
How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
- Q:-
A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of solution is 1.2 g mL-1, then what shall be the molarity of the solution?
- Q:-
Henry's law constant for CO2 in water is 1.67 x 108Pa at 298 K. Calculate the quantity of CO2in 500 mL of soda water when packed under 2.5 atm CO2 pressure at 298 K.
- Q:-
Calculate the mass of a non-volatile solute (molar mass 40 g mol-1) which should be dissolved in 114 g octane to reduce its vapour pressure to 80%.
- Q:-
The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively, at 350 K. Find out the composition of the liquid mixture if total vapour pressure is 600 mm Hg. Also find the composition of the vapour phase.
- Q:-
Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
- Q:-
How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of both?
- Q:-
If NaCl is doped with 10-3mol % of SrCl2, what is the concentration of cation vacancies?
Recently Viewed Questions of Class 12 Chemistry
- Q:-
Write the structures of products of the following reactions;
(i)

(ii)

(iii)

(iv)

- Q:-
Classify the following solids in different categories based on the nature of intermolecular forces operating in them:
Potassium sulphate, tin, benzene, urea, ammonia, water, zinc sulphide, graphite, rubidium, argon, silicon carbide. - Q:-
Define the term solution. How many types of solutions are formed? Write briefly about each type with an example.
- Q:-
Consider the reaction:
Cr2 O72- + 14H+ + 6e- → Cr3+ + 8H2O
What is the quantity of electricity in coulombs needed to reduce 1 mol of Cr2 O72-?
- Q:-
What are the oxidation states of phosphorus in the following:
(i) H3PO3
(ii) PCl3
(iii) Ca3P2
(iv) Na3PO4
(v) POF3?
- Q:-
Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
(ii) Benzene to m-bromophenol
(iii) Benzoic acid to aniline
(iv) Aniline to 2,4,6-tribromofluorobenzene
(v) Benzyl chloride to 2-phenylethanamine
(vi) Chlorobenzene to p-chloroaniline
(vii) Aniline to p-bromoaniline
(viii) Benzamide to toluene
(ix) Aniline to benzyl alcohol.
- Q:-
In a pseudo first order hydrolysis of ester in water, the following results were obtained:
t/s 0 30 60 90 [Ester]mol L - 1
0.55 0.31 0.17 0.085 (i) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(ii) Calculate the pseudo first order rate constant for the hydrolysis of ester.
- Q:-
Differentiate between globular and fibrous proteins.
- Q:-
The partial pressure of ethane over a solution containing 6.56 x 10-3 g of ethane is 1 bar. If the solution contains 5.00 x 10-2 g of ethane, then what shall be the partial pressure of the gas?
- Q:-
Define the following terms:
(i) Mole fraction (ii) Molality (iii) Molarity (iv) Mass percentage.
5 Comment(s) on this Question
Last-minute helppp.. really appreciateable.....!
Its nice,congratulations saralstudy
It is really helpful
Make something original
Most Useful page for a Indian Student.
- NCERT Chapter
