Giving examples, differentiate between 'roasting' and 'calcination'.
Roasting is the process of converting sulphide ores to oxides by heating the ores in a regular supply of air at a temperature below the melting point of the metal. For example, sulphide ores of Zn, Pb, and Cu are converted to their respective oxides by this process.

On the other hand, calcination is the process of converting hydroxide and carbonate ores to oxides by heating the ores either in the absence or in a limited supply of air at a temperature below the melting point of the metal. This process causes the escaping of volatile matter leaving behind the metal oxide. For example, hydroxide of Fe, carbonates of Zn, Ca, Mg are converted to their respective oxides by this process.
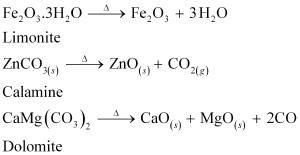
Popular Questions of Class 12 Chemistry
- Q:-
Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone
(vii) Ethanal and Propanal
- Q:-
A 5% solution (by mass) of cane sugar in water has freezing point of 271 K. Calculate the freezing point of 5% glucose in water if freezing point of pure water is 273.15 K.
- Q:-
How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
- Q:-
A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of solution is 1.2 g mL-1, then what shall be the molarity of the solution?
- Q:-
Henry's law constant for CO2 in water is 1.67 x 108Pa at 298 K. Calculate the quantity of CO2in 500 mL of soda water when packed under 2.5 atm CO2 pressure at 298 K.
- Q:-
Calculate the mass of a non-volatile solute (molar mass 40 g mol-1) which should be dissolved in 114 g octane to reduce its vapour pressure to 80%.
- Q:-
The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively, at 350 K. Find out the composition of the liquid mixture if total vapour pressure is 600 mm Hg. Also find the composition of the vapour phase.
- Q:-
Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
- Q:-
How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of both?
- Q:-
If NaCl is doped with 10-3mol % of SrCl2, what is the concentration of cation vacancies?
Recently Viewed Questions of Class 12 Chemistry
- Q:-
The partial pressure of ethane over a solution containing 6.56 x 10-3 g of ethane is 1 bar. If the solution contains 5.00 x 10-2 g of ethane, then what shall be the partial pressure of the gas?
- Q:-
How will you convert?
(i) Benzene into aniline
(ii) Benzene into N, N-dimethylaniline
(iii) Cl-(CH2)4-Cl into hexan-1, 6-diamine?
- Q:-
Why are powdered substances more effective adsorbents than their crystalline forms?
- Q:-
Which compound in each of the following pairs will react faster in SN2 reaction with OH-?
(i) CH3Br or CH3I
(ii) (CH3)3CCl or CH3Cl
- Q:-
State Henry's law and mention some important applications?
- Q:-
If NaCl is doped with 10-3mol % of SrCl2, what is the concentration of cation vacancies?
- Q:-
Depict the galvanic cell in which the reaction Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s) takes place.
Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
- Q:-
Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
- Q:-
Give the uses of freon 12, DDT, carbon tetrachloride and iodoform.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- NCERT Chapter
