Class 12 Chemistry - Chapter Chemical Kinetics NCERT Solutions | The time required for 10% completion of
The time required for 10% completion of a first order reaction at 298 K is equal to that required for its 25% completion at 308 K. If the value of A is 4 x 1010 s-1. Calculate k at 318 K and Ea.
For a first order reaction,
t = 2.303 / k log a / a - x
At 298 K,
t = 2.303 / k log 100 / 90
= 0.1054 / k
At 308 K,
t' = 2.303 / k' log 100 / 75
= 2.2877 / k'
According to the question,
t = t'
⇒ 0.1054 / k = 2.2877 / k'
⇒ k' / k = 2.7296
From Arrhenius equation,we obtain
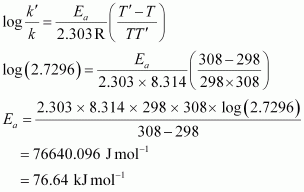
To calculate k at 318 K,
It is given that, A = 4 x 1010 s-1, T = 318K
Again, from Arrhenius equation, we obtain

Therefore, k = Antilog (-1.9855)
= 1.034 x 10-2 s -1
More Questions From Class 12 Chemistry - Chapter Chemical Kinetics
- Q:-
The half-life for radioactive decay of 14C is 5730 years. An archaeological artifact containing wood had only 80% of the 14C found in a living tree. Estimate the age of the sample.
- Q:-
For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction.
- Q:-
The rate of a reaction quadruples when the temperature changes from 293 K to 313 K. Calculate the energy of activation of the reaction assuming that it does not change with temperature.
- Q:-
A first order reaction takes 40 min for 30% decomposition. Calculate t1/2.
- Q:-
In a reaction, 2A → Products, the concentration of A decreases from 0.5 mol L-1 to 0.4 mol L-1 in 10 minutes. Calculate the rate during this interval?
- Q:-
The conversion of molecules X to Y follows second order kinetics. If concentration of X is increased to three times how will it affect the rate of formation of Y?
- Q:-
During nuclear explosion, one of the products is 90Sr with half-life of 28.1 years. If 1μg of 90Sr was absorbed in the bones of a newly born baby instead of calcium, how much of it will remain after 10 years and 60 years if it is not lost metabolically.
- Q:-
Sucrose decomposes in acid solution into glucose and fructose according to the first order rate law, with t1/2 = 3.00 hours. What fraction of sample of sucrose remains after 8 hours?
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
The decomposition of A into product has value of k as 4.5 x 103 s-1 at 10°C and energy of activation 60 kJ mol-1. At what temperature would k be 1.5 x 104 s-1?
Popular Questions of Class 12 Chemistry
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
- Q:-
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Recently Viewed Questions of Class 12 Chemistry
- Q:-
A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of solution is 1.2 g mL-1, then what shall be the molarity of the solution?
- Q:-
What is a biodegradable polymer? Give an example of a biodegradable aliphatic polyester.
- Q:-
Write short notes on the following:
(i) Carbylamine reaction (ii) Diazotisation
(iii) Hofmann's bromamide reaction (iv) Coupling reaction
(v) Ammonolysis (vi) Acetylation
(vii) Gabriel phthalimide synthesis.
- Q:-
Write the mechanism of the reaction of HI with methoxymethane.
- Q:-
Calculate the efficiency of packing in case of a metal crystal for
(i) simple cubic
(ii) body-centred cubic
(iii) face-centred cubic (with the assumptions that atoms are touching each other).
- Q:-
Write the monomers used for getting the following polymers. (i) Polyvinyl chloride (ii) Teflon (iii) Bakelite
- Q:-
How are polymers classified on the basis of structure?
- Q:-
Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
- Q:-
Define the term solution. How many types of solutions are formed? Write briefly about each type with an example.
- Q:-
Calculate the 'spin only' magnetic moment of M2+(aq) ion (Z = 27).
4 Comment(s) on this Question
Wonderful. I appreciate your efforts. But you can make this visually appealing too.
Helps me a lot
Thanks for clearing my doubts
I thought it's very complicated but it is much easier....thanks for clearing my doubts...
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
