Class 12 Chemistry - Chapter Biomolecules NCERT Solutions | How do you explain the amphoteric behavi
How do you explain the amphoteric behaviour of amino acids?
In aqueous solution, the carboxyl group of an amino acid can lose a proton and the amino group can accept a proton to give a dipolar ion known as zwitter ion.

Therefore, in zwitter ionic form, the amino acid can act both as an acid and as a base.


Thus, amino acids show amphoteric behaviour.
More Questions From Class 12 Chemistry - Chapter Biomolecules
- Q:-
What happens when D-glucose is treated with the following reagents? (i)HI (ii)Bromine water (iii)HNO3
- Q:-
How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
- Q:-
The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
- Q:-
Define the following as related to proteins
(i) Peptide linkage (ii) Primary structure (iii) Denaturation.
- Q:-
What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
- Q:-
The two strands in DNA are not identical but are complementary. Explain.
- Q:-
What are nucleic acids? Mention their two important functions.
- Q:-
Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
- Q:-
Where does the water present in the egg go after boiling the egg?
Popular Questions of Class 12 Chemistry
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Recently Viewed Questions of Class 12 Chemistry
- Q:-
Why is sulphuric acid not used during the reaction of alcohols with KI?
- Q:-
How many lattice points are there in one unit cell of each of the following lattice?
(i) Face-centred cubic
(ii) Face-centred tetragonal
(iii) Body-centred
- Q:-
Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(i) 1-Bromo-1-methylcyclohexane
(ii) 2-Chloro-2-methylbutane
(iii) 2,2,3-Trimethyl-3-bromopentane.
- Q:-
Vapour pressure of pure water at 298 K is 23.8 mm Hg. 50 g of urea (NH2CONH2) is dissolved in 850 g of water. Calculate the vapour pressure of water for this solution and its relative lowering.
- Q:-
Complete each synthesis by giving missing starting material, reagent or products
(i)
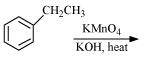
(ii)

(iii)

(iv)

(v)

(vi)
- Q:-
How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
- Q:-
Arrange each set of compounds in order of increasing boiling points.
(i) Bromomethane, Bromoform, Chloromethane, Dibromomethane.
(ii) 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
- Q:-
How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of both?
- Q:-
How are the following conversions carried out?
(i) Propene → Propan-2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl magnesium chloride → Propan-1-ol.
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol.
- Q:-
State Henry's law and mention some important applications?
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
