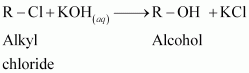A:
(i)

An SN2 reaction involves the approaching of the nucleophile to the carbon atom to which the leaving group is attached. When the nucleophile is sterically hindered, then the reactivity towards SN2 displacement decreases. Due to the presence of substituents, hindrance to the approaching nucleophile increases in the following order.
1-Bromopentane < 2-bromopentane < 2-Bromo-2-methylbutane
Hence, the increasing order of reactivity towards SN2 displacement is:
2-Bromo-2-methylbutane < 2-Bromopentane < 1-Bromopentane
(ii)

Since steric hindrance in alkyl halides increases in the order of 1° < 2° < 3°, the increasing order of reactivity towards SN2 displacement is 3° < 2° < 1°.
Hence, the given set of compounds can be arranged in the increasing order of their reactivity towards SN2 displacement as:
2-Bromo-2-methylbutane < 2-Bromo-3-methylbutane < 1-Bromo-3-methylbutane
[2-Bromo-3-methylbutane is incorrectly given in NCERT]
(iii)

The steric hindrance to the nucleophile in the SN2 mechanism increases with a decrease in the distance of the substituents from the atom containing the leaving group. Further, the steric hindrance increases with an increase in the number of substituents. Therefore, the increasing order of steric hindrances in the given compounds is as below:
1-Bromobutane < 1-Bromo-3-methylbutane < 1-Bromo-2-methylbutane
< 1-Bromo-2, 2-dimethylpropane
Hence, the increasing order of reactivity of the given compounds towards SN2 displacement is:
1-Bromo-2, 2-dimethylpropane < 1-Bromo-2-methylbutane < 1-Bromo-3- methylbutane < 1-Bromobutane