Class 12 Chemistry - Chapter The Solid State NCERT Solutions | What type of defect can arise when a sol
What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
When a solid is heated, vacancy defect can arise. A solid crystal is said to have vacancy defect when some of the lattice sites are vacant.
Vacancy defect leads to a decrease in the density of the solid.
More Questions From Class 12 Chemistry - Chapter The Solid State
- Q:-
If NaCl is doped with 10-3mol % of SrCl2, what is the concentration of cation vacancies?
- Q:-
A cubic solid is made of two elements P and Q. Atoms of Q are at the corners of the cube and P at the body-centre. What is the formula of the compound? What are the coordination numbers of P and Q?
- Q:-
An element with molar mass 2.7 x 10-2kg mol-1 forms a cubic unit cell with edge length 405 pm. If its density is 2.7 x 103 kg m-3, what is the nature of the cubic unit cell?
- Q:-
Copper crystallises into a fcc lattice with edge length 3.61 x 10-8cm. Show that the calculated density is in agreement with its measured value of 8.92 g cm-3.
- Q:-
A compound is formed by two elements M and N. The element N forms ccp and atoms of M occupy 1/3rd of tetrahedral voids. What is the formula of the compound?
- Q:-
Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 125 pm.
(i) What is the length of the side of the unit cell?
(ii) How many unit cells are there in 1.00 cm3of aluminium?
- Q:-
Analysis shows that nickel oxide has the formula Ni0.98 O1.00. What fractions of nickel exist as Ni2+and Ni3+ions?
- Q:-
Distinguish between
(i)Hexagonal and monoclinic unit cells
(ii) Face-centred and end-centred unit cells.
- Q:-
Solid A is a very hard electrical insulator in solid as well as in molten state and melts at extremely high temperature. What type of solid is it?
- Q:-
Ionic solids, which have anionic vacancies due to metal excess defect, develop colour. Explain with the help of a suitable example.
Popular Questions of Class 12 Chemistry
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
- Q:-
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Recently Viewed Questions of Class 12 Chemistry
- Q:-
What is meant by 'disproportionation'? Give two examples of disproportionation reaction in aqueous solution.
- Q:-
How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
- Q:-
Complete each synthesis by giving missing starting material, reagent or products
(i)
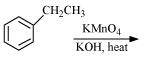
(ii)

(iii)

(iv)

(v)

(vi)
- Q:-
Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(i) 1-Bromo-1-methylcyclohexane
(ii) 2-Chloro-2-methylbutane
(iii) 2,2,3-Trimethyl-3-bromopentane.
- Q:-
[NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why?
- Q:-
Explain the following with an example.
(i) Kolbe's reaction.
(ii) Reimer-Tiemann reaction.
(iii) Williamson ether synthesis.
(iv) Unsymmetrical ether.
- Q:-
What are the monomeric repeating units of Nylon-6 and Nylon-6, 6?
- Q:-
What happens when H3PO3 is heated?
- Q:-
Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(i) Fe3+(aq) and I-(aq)
(ii) Ag+ (aq) and Cu(s)
(iii) Fe3+ (aq) and Br- (aq)
(iv) Ag(s) and Fe3+ (aq)
(v) Br2 (aq) and Fe2+ (aq).
- Q:-
What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
2 Comment(s) on this Question
Nice
This is not the proper answer for this question
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
